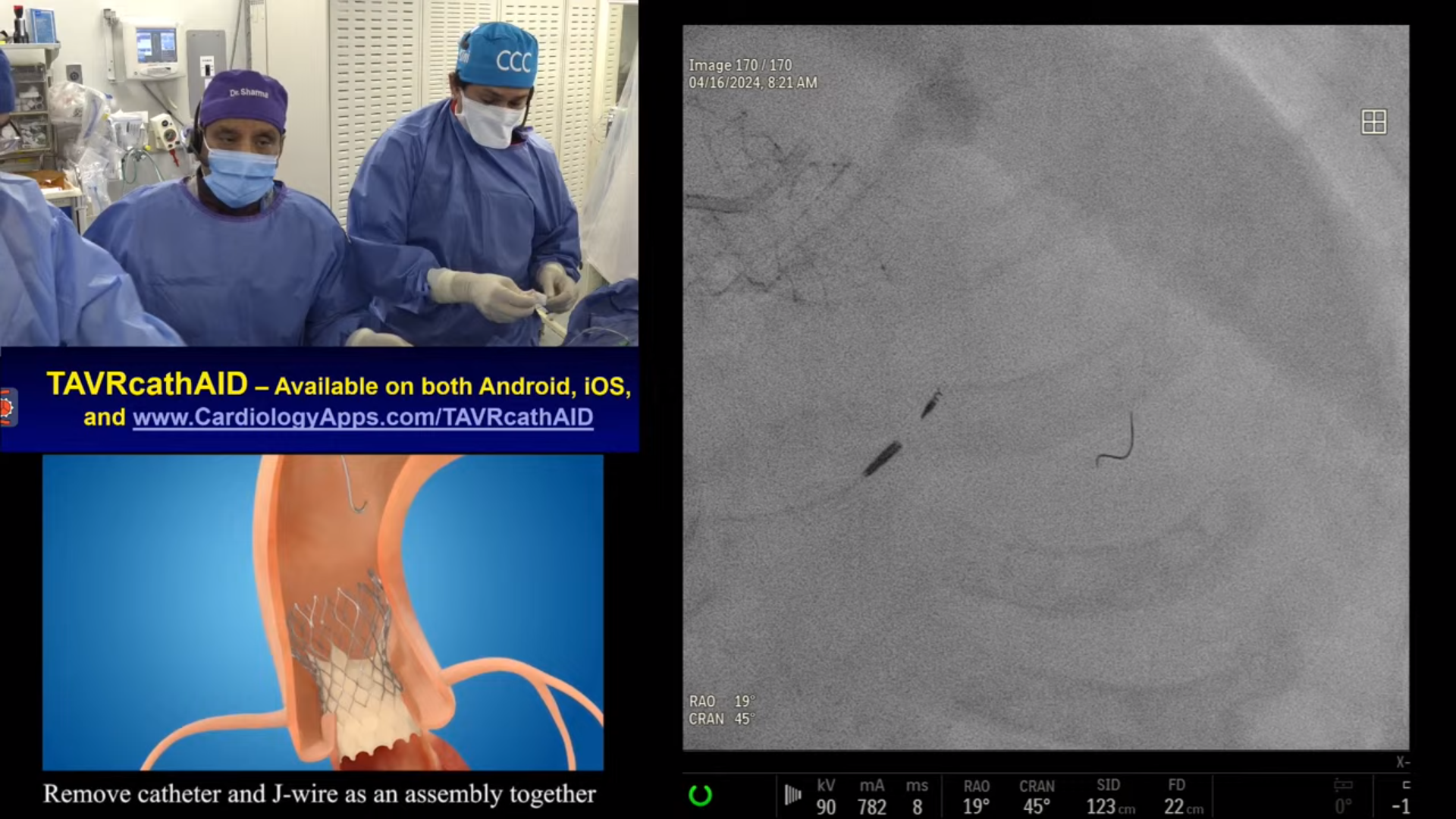
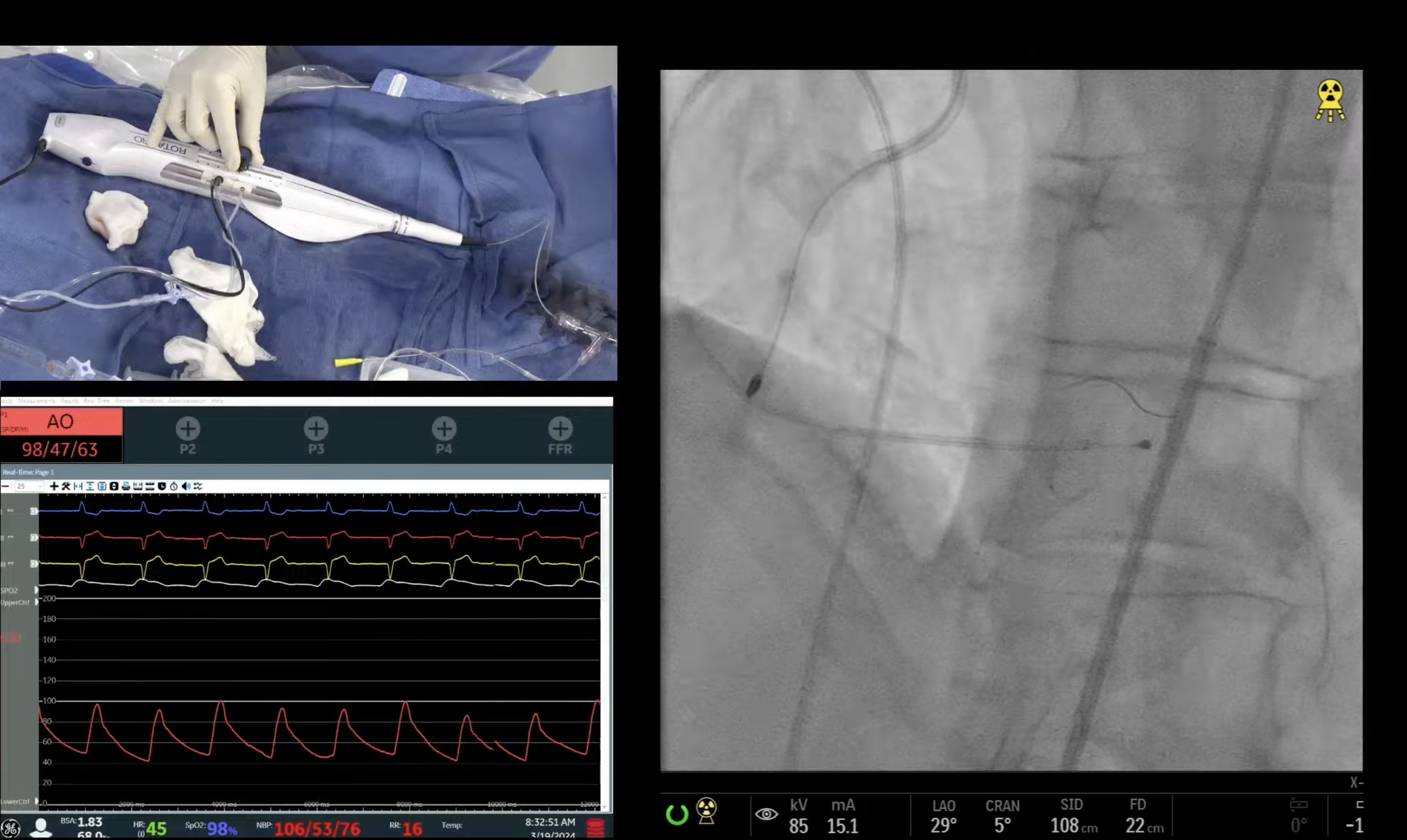
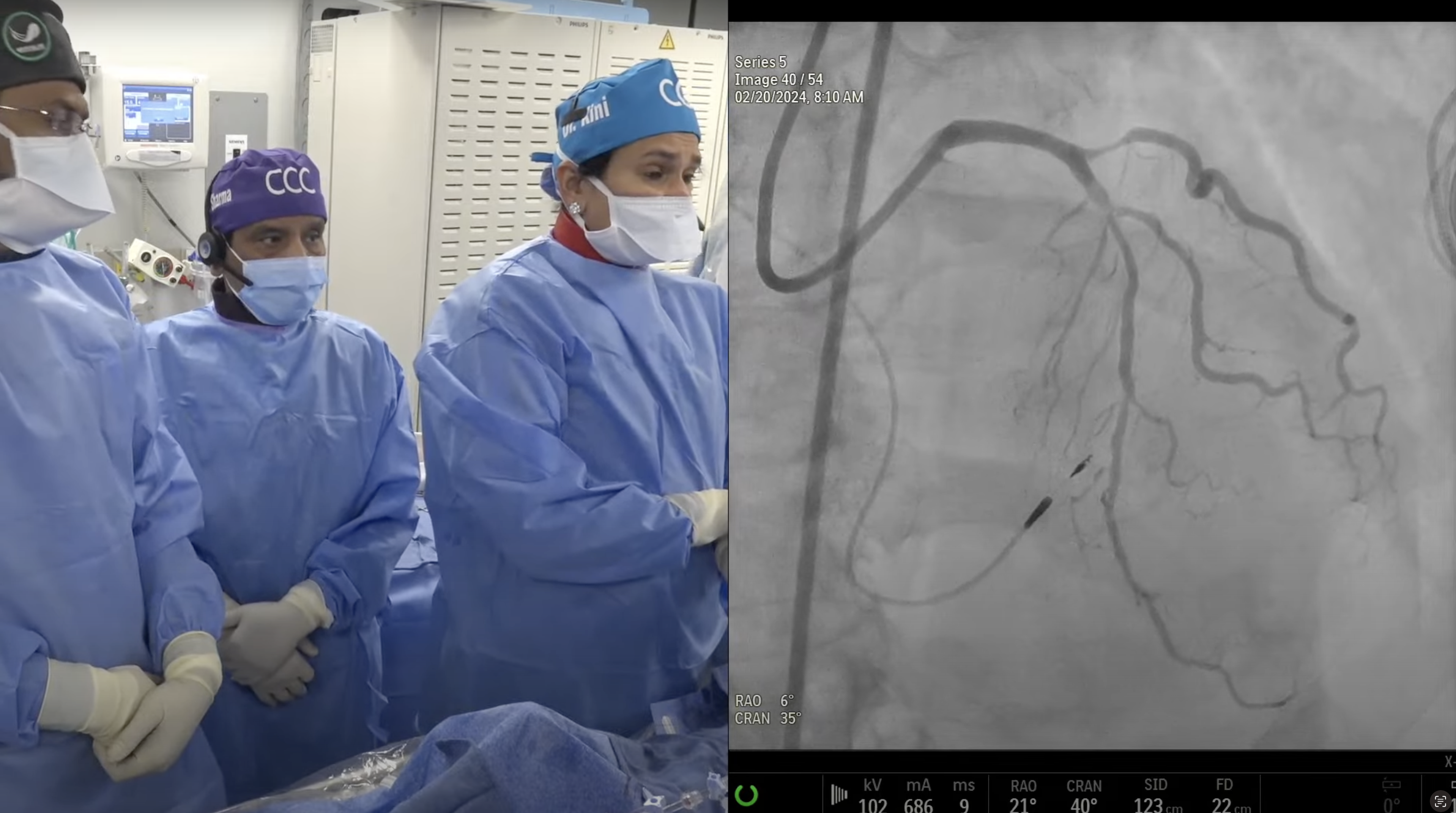
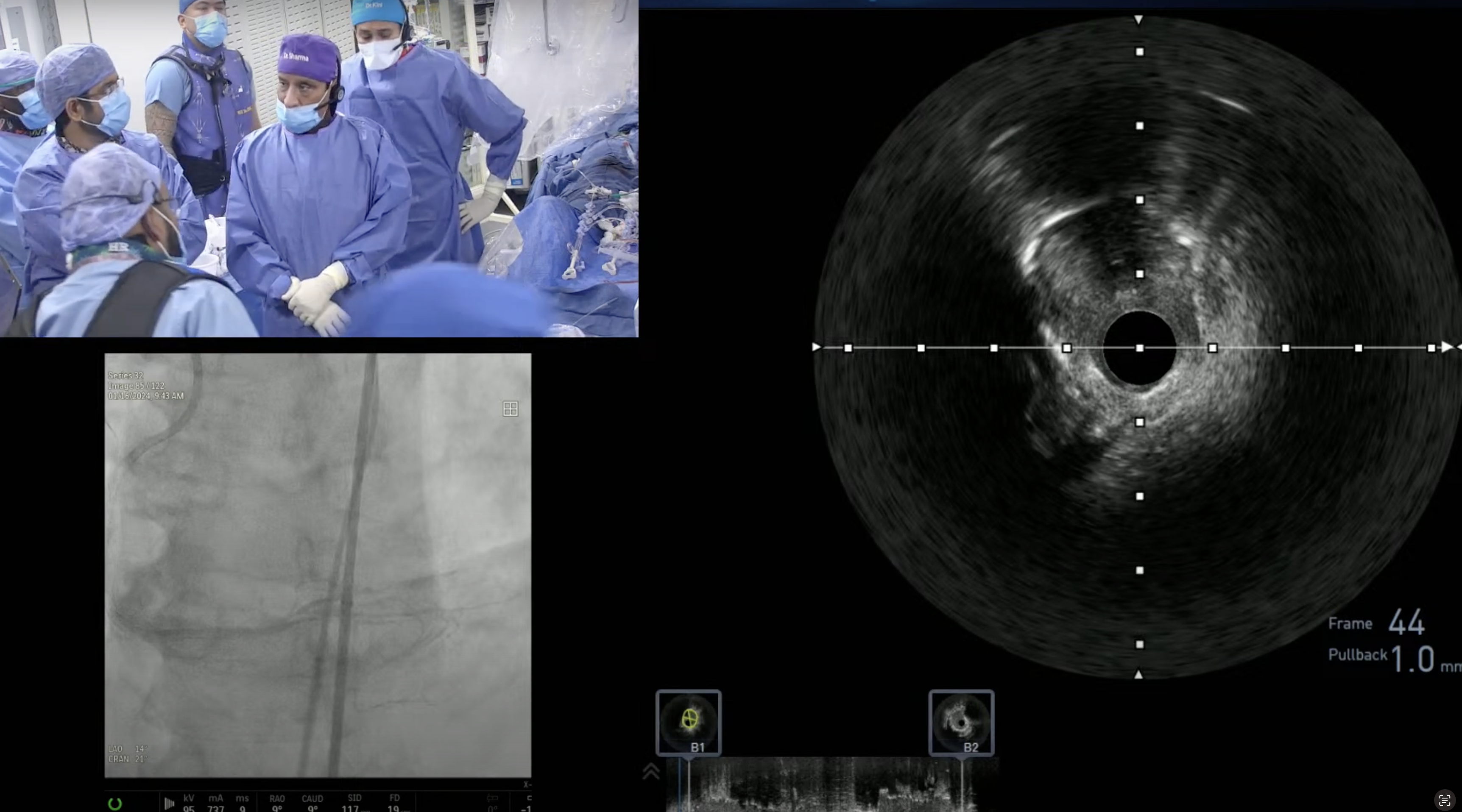
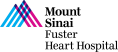Case and Plan:
78 year-old-male presented on August 7, 2020 with progressive exertional dyspnea, NYHA class III. Workup revealed severe AS (AVA 0.7cm2), normal LV function and STS mortality of 1.1%. After heart team discussion, patient underwent successful TAVR using 26mm SAPIEN-3 Ultra with excellent results, AV area of 2.0cm2 and no PVL. A coronary angiography pre-TAVR procedure revealed severe calcific 2 V CAD of LAD (iFR 0.79) and RPDA with SYNTAX score of 22. Patient continued to have mild DOE and chest pressure likely due to anatomically & physiologically significant CAD of LAD lesion and possibly RPDA lesion. Now planned post TAVR, for imaging guided orbital atherectomy of long calcified LAD using trans-radial access.
Q&A
Q
How is the volume of the procedures at your institution presently?
A.
It seems that all the clinic visits, non-invasive and invasive volume have come back to preCovid volume; Cath lab volume of this month will be 100% of the Sept 2019 volume. EP volume is only lagging behind to about 90%.
Q
Why did they exclude LMCA cases in Complete TAVR trial?
A.
Reason to exclude LM lesions in Complete TAVR trial was known high mortality of LM CAD on MMT. One arm of Complete TAVR is MMT and other arm being PCI. Similar exclusion was done In the Ischemia trial also excluding pts with LM disease on CTA.
Q
Do you think TAILOR PCI is the death nail of genomic testing?
A.
I guess so, because we use potent P2Y12 inhibitors on clinical grounds to minimize ST for few months and deescalate in many pts after 3-6 mths due to fear of bleeding. Tailor PCI trial showed that white genome guided PCI caused slightly less ischemic events but also caused more bleeding events. Hence there will be no new recommendation in this field.
Q
Would you foresee any situation at all to use genomic testing although it is clear there is no role for routine testing?
A.
I personally have not seen any clinical scenario where antiplatelet therapy choice requires genome testing. We still do Accumetrics PRU assay in situation where high bleeding risk pts (even already on clopidogrel) require complex lesion (like LM, bifurcation, use of 3+ stents) PCI. In these pts (which makes about 10%), we do PRU testing on the day of PCI (if on maintenance clopidogrel) or next day (if loaded on previous day). If PRU is >230, then unless absolute contraindication, switch to Prasugrel 5mg daily (after 30mg LD) or to Ticagrelor 90mg twice daily (after 180mg load). These pts routinely get PPI and abbreviated duration of aspirin 1-3mths).
Q
Which situations do you like the Akari guide?
A.
Akari guides (usually AK 1.0) are very good for RCA PCI via Radial approach and for both RCA or Left System PCI in pts with Evolut TAVR valve.
Q
Broadly speaking, accessing coronaries is always easier with Sapien valve?
A.
Yes it is the correct statement that coronary access is much easier after Sapien TAVR vs Evolut CoreValve TAVR. We have published that commisural tab alignment orientation at the time of valve deployment, makes coronary access easier in future. But in many cases, we still struggle for coronary engagement after Evolut valve.
Q
Always 6F to access TAVR related coronaries?
A.
Yes most PCIs post TAVR are done using 6Fr access. Rare cases of LM bifurcation PCI using 2 stents or heavily calcified lesion requiring 2mm Rota burr, will get 7Fr vascular access.
Q
Which situations will you use 10 mg Prasugrel?
A.
Pts with over 100Kg weight, use of 3+ DES, known stent thrombosis and known clopidogrel nonresponder (not at high bleeding risk), routinely get 10mg Prasugrel daily.
Q
Your use of Ticagrelor is lower across the board?
A.
Particularly after ISAR REACT-5 trial our Prasugrel use has increased and Ticagrelor use has declined by 50%.
Q
Ticagrelor was supposed to be safer for elderly and Prasugrel more risky - present answer to this situation now?
A.
In elderly (>75-80yrs old) safest is clopidogrel followed by Ticagrelor with aspirin for 1-3 mths and then Prasugrel 5mg daily.