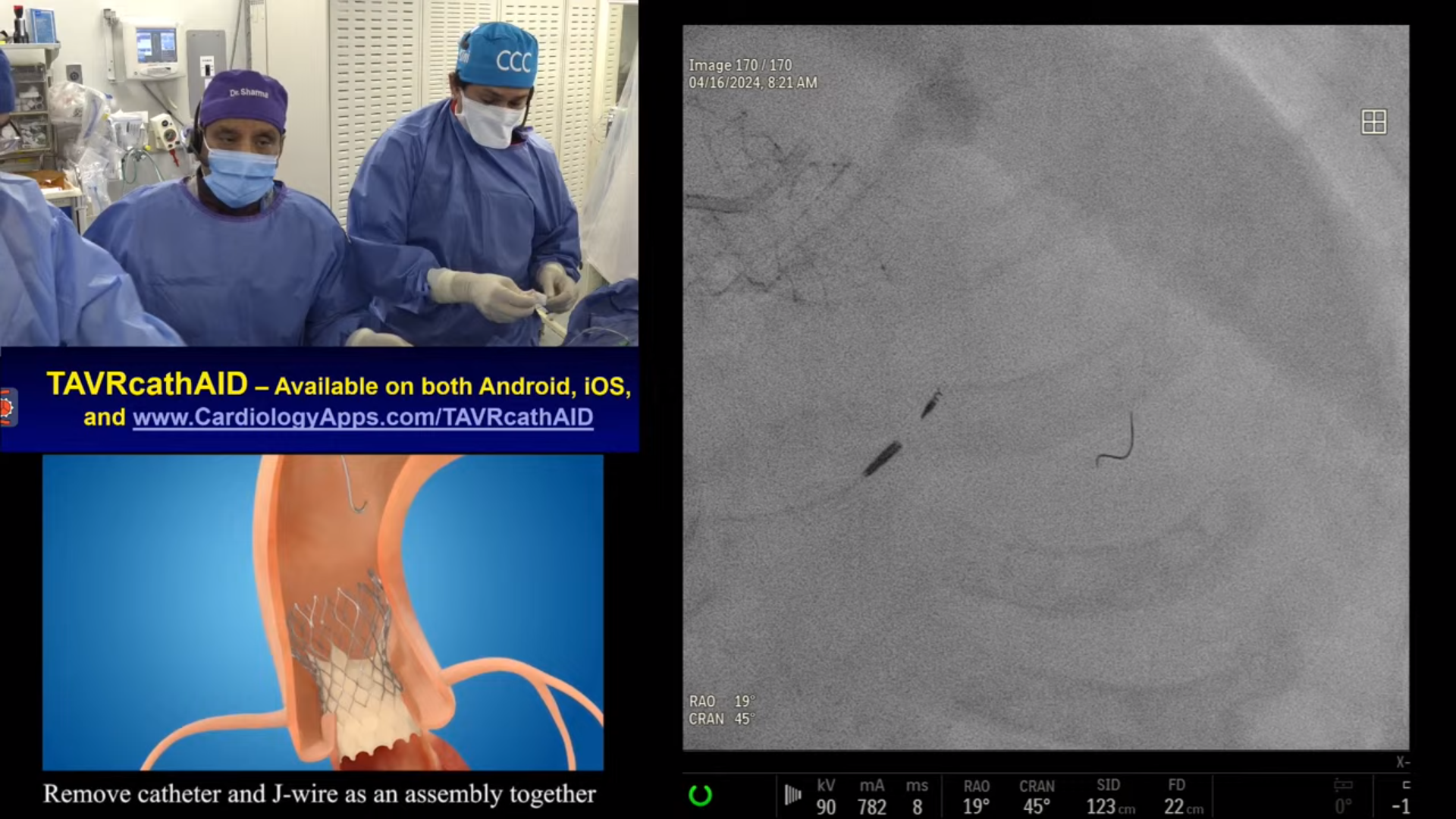
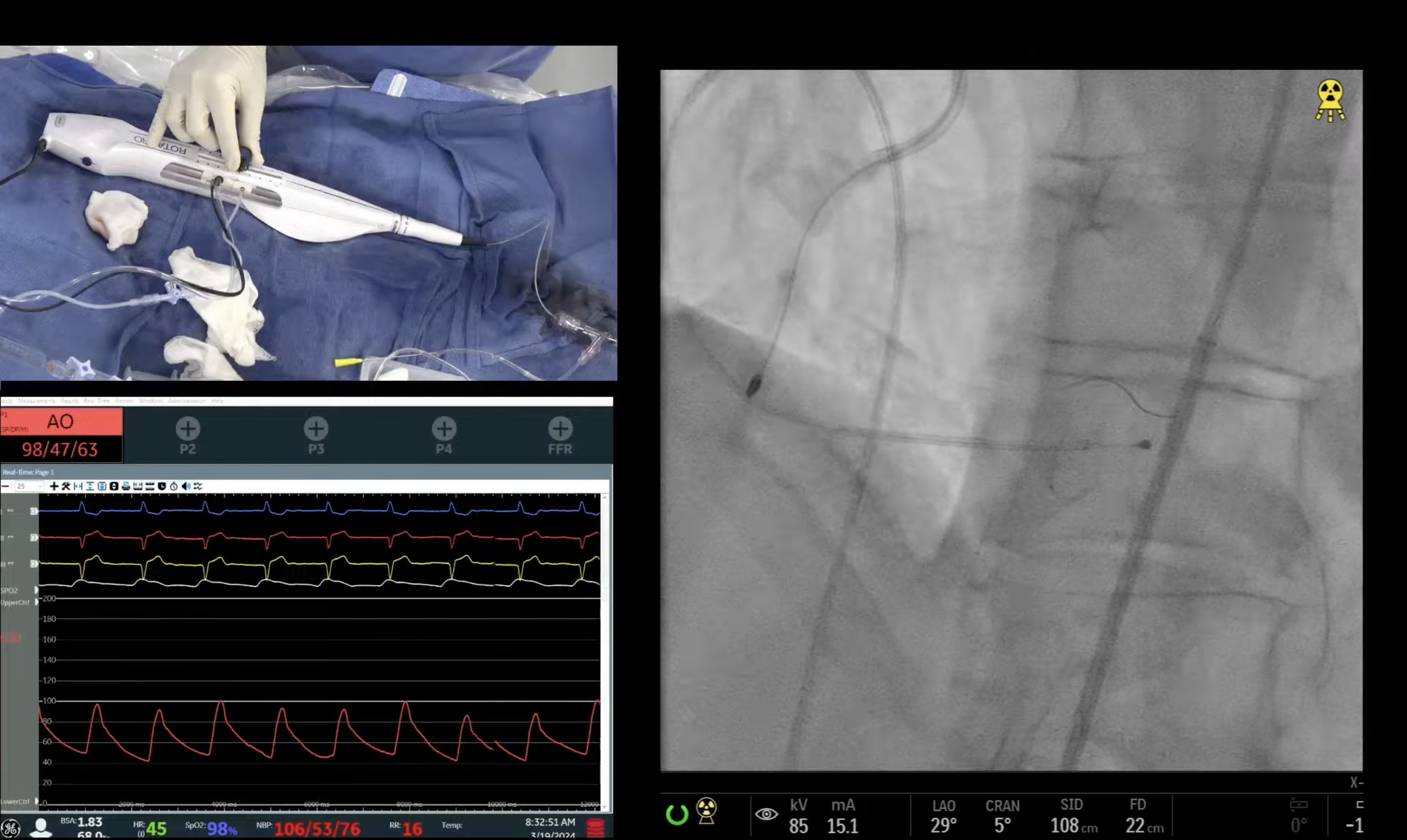
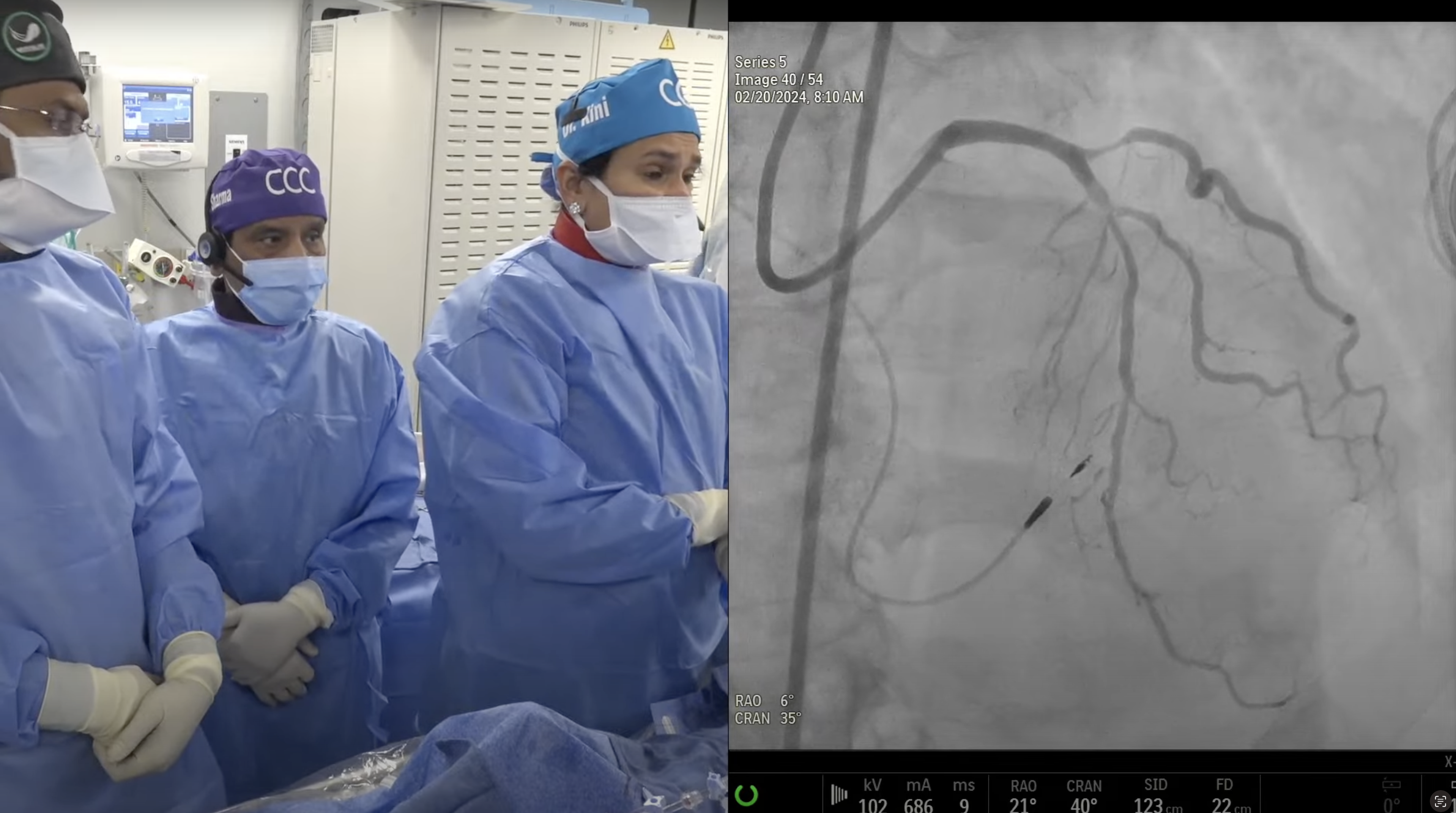
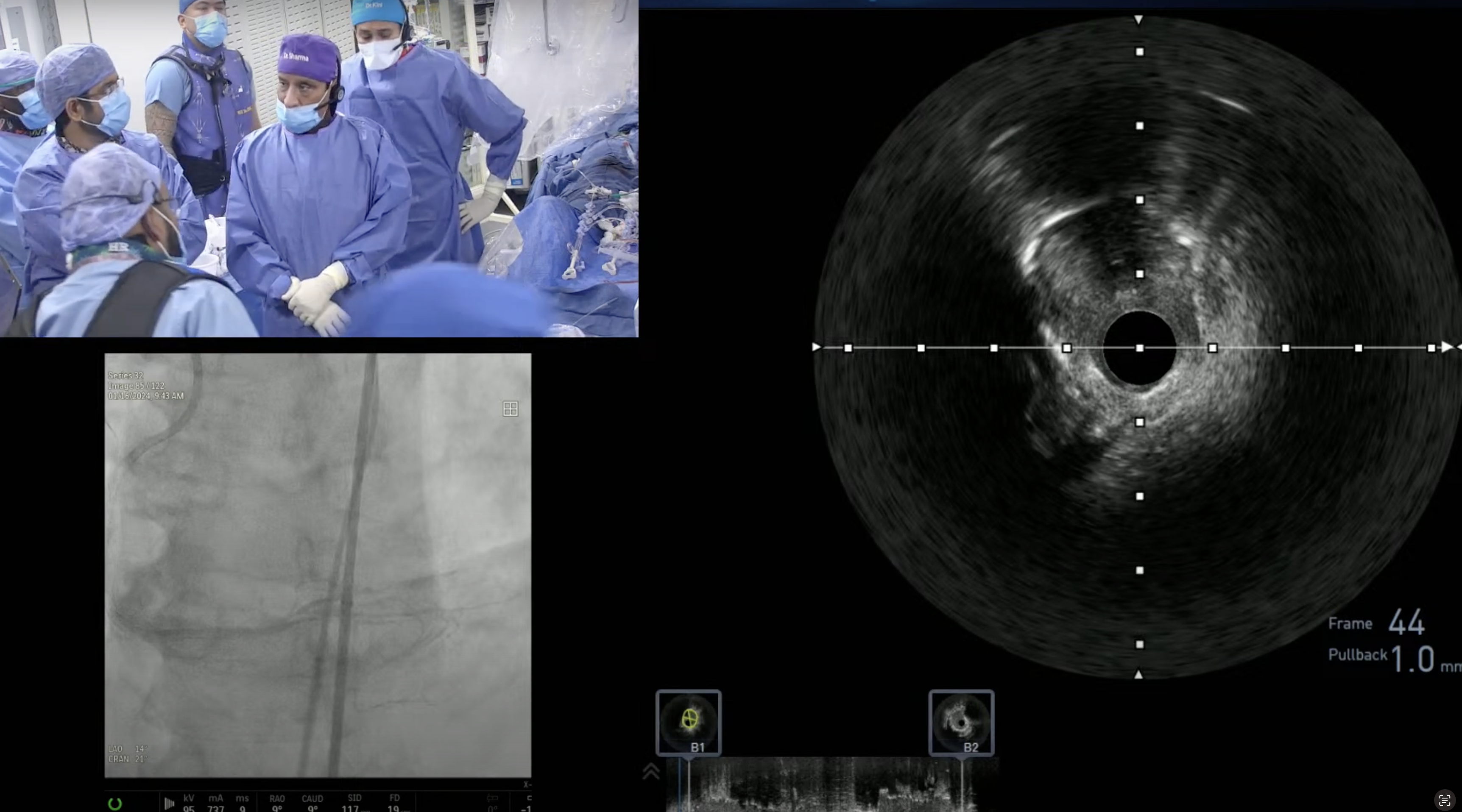
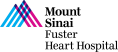CASE & Plan:
69-year-old male with cirrhosis presented with CCS Class III angina. A Cardiac Cath at OSH on December 27, 2022 revealed extensive 3 V CAD: multiple calcified RCA lesions with ISR of RPDA and RPL1, trifurcation lesion of 90% proximal LAD, 90% LAD-D1, 70% proximal LCx-OM1, 80% Ramus with SYNTAX Score of 39 and LVEF 60%. After Heart Team discussion, CABG was declined due to liver cirrhosis and recommended high-risk multivessel PCI. Patient underwent successful PCI of multiple RCA lesions using three Xience Skypoint DES and did well. Patient is now planned for imaging guided complex PCI of LAD/LCx/Ramus trifurcation.
Q&A
Q
Do you normally go up to 6 ATMs on the IVL balloon? We have been using 4 ATMs?
A.
That is correct, initial IVL balloon inflation pressure is 4 ATM for 10 sec and then go to 6 ATM for 2 seconds before deflating. We do go upto 6 ATM many times when feel that lesion has not fully yielded.
Q
Has CO2 accumulation been any problem in any case?
A.
CO2 accumulation has not been problem in any cases.
Q
IVL appears a particularly good strategy for under expanded stents?
A.
Fully agree that IVL should be the first line treatment for underexpanded stents followed by ELCA and then Rota.
Q
Could this case have been done with IVL only? Perhaps using a high pressure balloon for preparation?
A.
Yes present case could entirely have been done with IVL after balloon pre dilatation and if needed post dilatation with NC balloon.
Q
What is your experience with Orbital Atherectomy (OA) in such cases?
A.
We have seen that OA does not work in underexpanded stents; perhaps due to lack of heat generation, which is the predominant mechanism of RA in these cases.
Q
And with the excimer laser (ELCA)?
A.
Before IVL, ELCA used to be the first line treatment for underexpanded stents and works very well in 95% of cases. If fails, we use RA in conjunction or as the second line therapy.
Q
As per new ACC recommendations regarding imaging, do you perceive an increase in both IVUS and OCT? Or more with either?
A.
I predict that going forward both IVUS and OCT use will increase (2-3x of current volume). IVUS due to its ease of use, no need for contrast and easy interpretation will have the edge over OCT during PCI. Currently as per ACC-NCDR data, both together are used in 16-18% of PCIs in USA; 2/3rd IVUS and 1/3rd OCT.
Q
What would be the various implications of Yellow 3? Seems highly promising?
A.
Two most important implications of Yellow-3 are; almost 60% of stable CAD pts have TCFA and about 20% of pts do not have increase in cap thickness or lipid plaque burden despite significant decrease in LDL; Non-responders. We hope that genetic analysis of Yellow-3 will help to identify gene involved in this subset of non-responder pts.
Q
For the device to get FDA approval for its numerous indications, would a multicenter trial be mandatory?
A.
Yes it is correct that to get any device approved by FDA, a multi-center trial including 109+ pts will be needed; even as the single arm registry (all CTO devices were approved this way).
Q
How are Apps developed at your institution changing the interventional cardiology landscape?
A.
Our numerous Mount Sinai Apps for interventional medical education have been a great hit being downloaded and used globally; over 0.5M downloads so far.