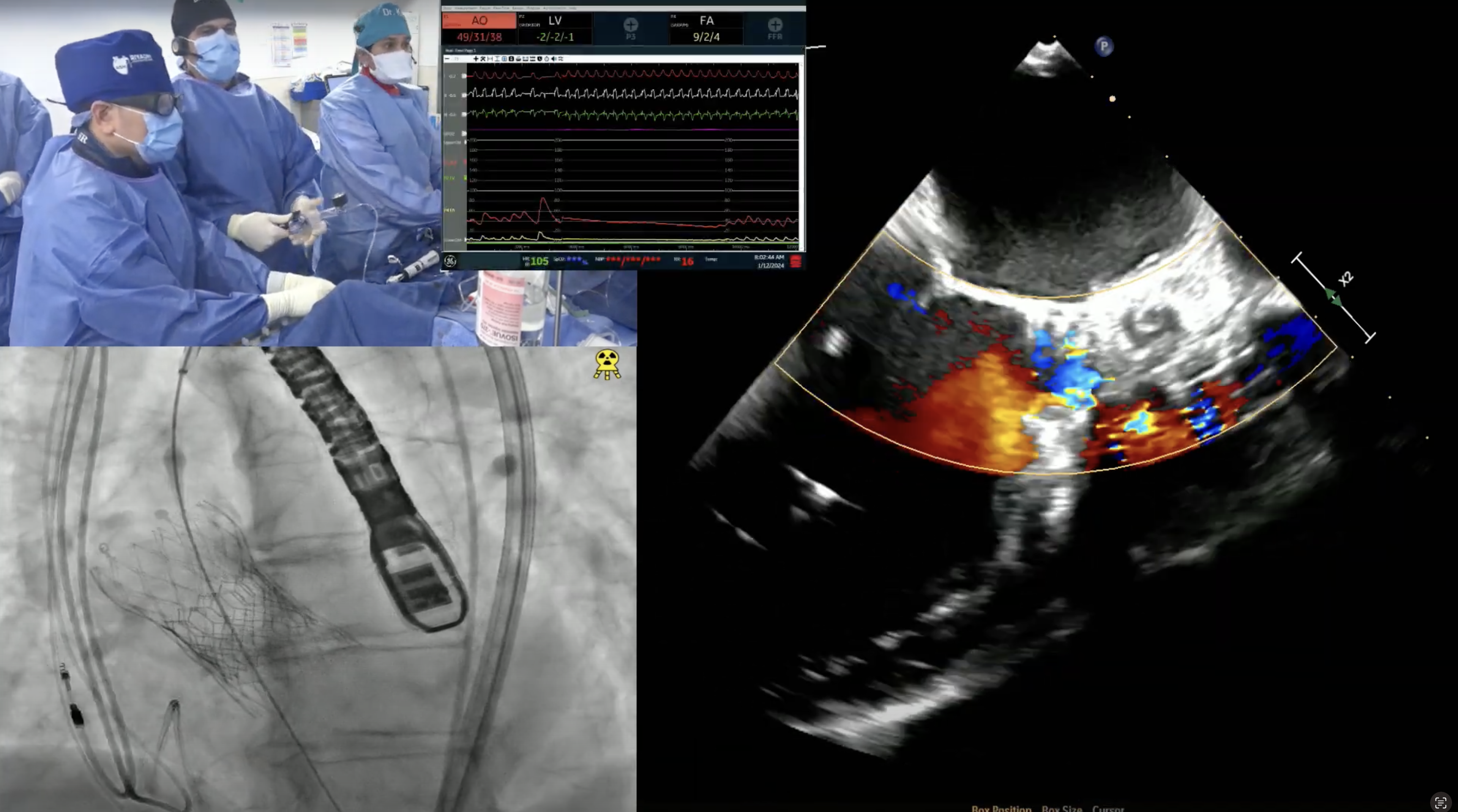
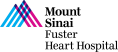Case & Plan: 77-year-old male presents with worsening lightheadedness and dyspnea on exertion. Past medical history is significant for hypertension, hyperlipidemia, non-obstructive CAD, AF, prior stroke with left sided weakness, s/p bioprosthetic aortic valve replacement in 2008 and H/o bladder cancer s/p resection (2018), stomach cancer s/p partial gastrectomy (2010) & prostate cancer s/p brachytherapy (2007). A recent echocardiogram revealed severe bioprosthetic AS/AR of prior 25mm Mosaic valve (PG/MG/PV: 67/42/4.1), mild MR, mild TR and LVEF 65%. CTA measurement revealed patient to be suitable for transfemoral (TF) valve-in-valve TAVR. The patient’s STS PROM risk for redo SAVR is 1.9%. Heart team evaluation deemed the patient at high risk for redo surgery and appropriate for TF TAVR. Now planned for TF valve-in-valve (ViV) TAVR via right percutaneous femoral arterial access using 26mm Evolut FX valve after balloon valve fracture using a 24mm True Balloon, with Sentinel cerebral embolic protection.