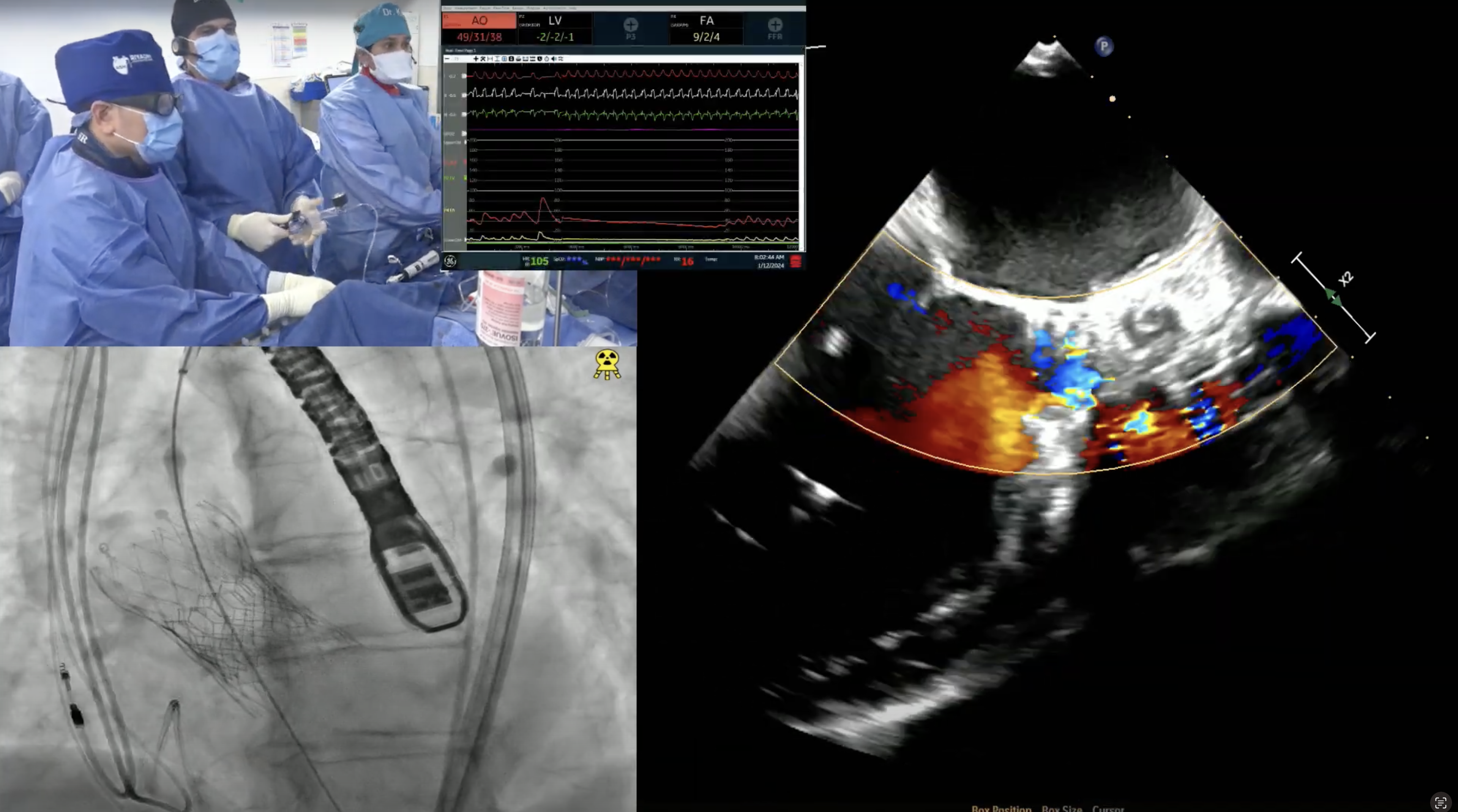
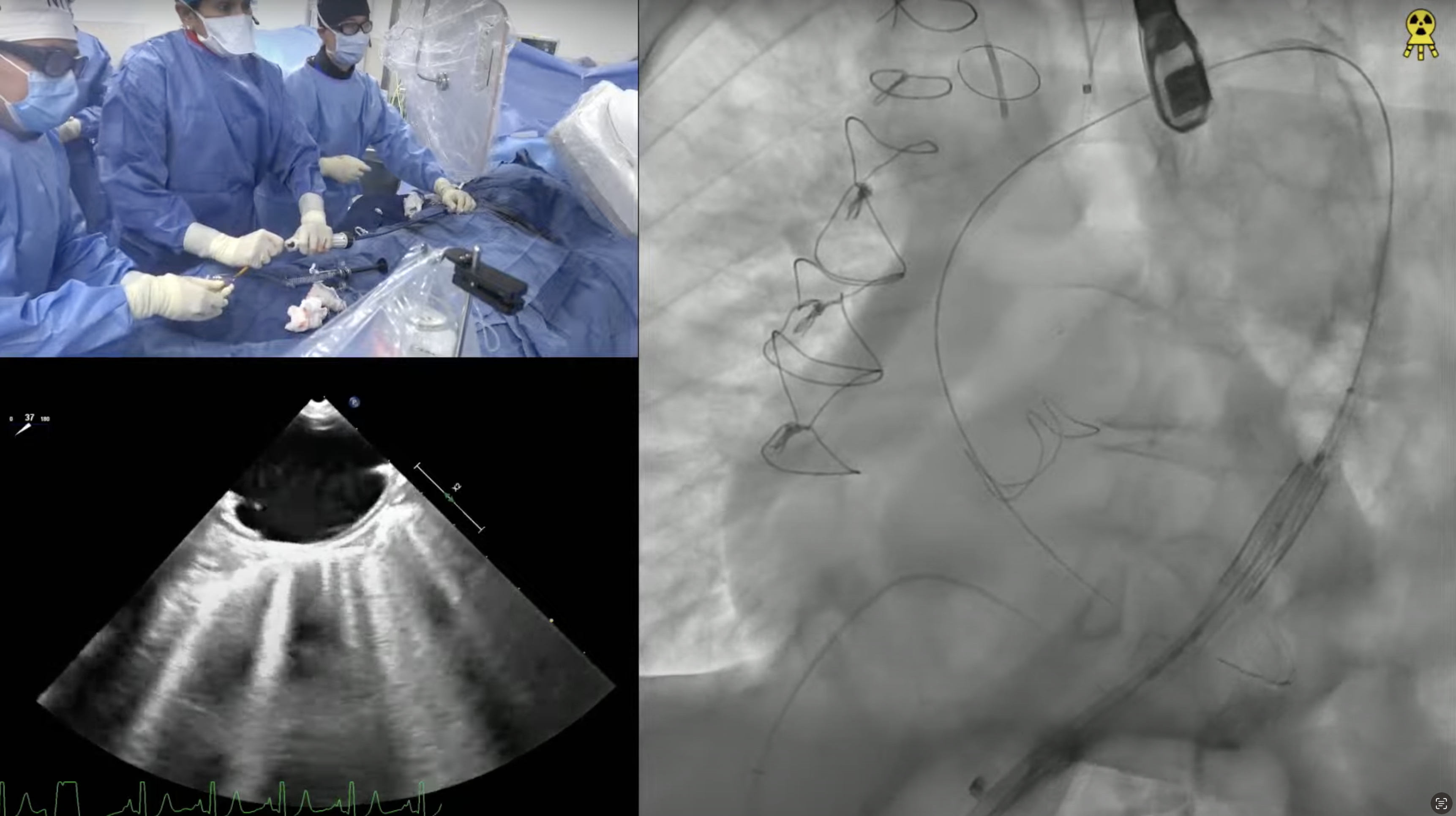
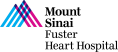An 85 year-old male presented with recent onset worsening of dyspnea on exertion and fatigue (NYHA III). His past history was significant for presence of hypertension, hyperlipidemia, idiopathic pulmonary fibrosis on steroids, Raynaud’s disease, BPH and hypothyroidism. He was known to have severe AS since 2014 and was medically managed. Recent TTE revealed severe valvular aortic stenosis; peak gradient = 65 mmHg, mean gradient = 41 mmHg, Doppler valve area = 0.79cm2, Ao peak velocity = 4.03m/sec and LVEF of 57 %. Coronary angiogram showed 60% mid LAD disease which was non-significant by FFR. CT angiogram revealed minimum diameters of 8.5mm for bilateral common iliac arteries and aortic annulus of 26.1 X 26.8 mm (average 26.5mm) with an annular circumference of 85mm. The STS mortality risk for Surgical AVR is 5.6% and the Logistic Euroscore mortality risk is 12.4%. Patient was determined to be high risk for SAVR due to age, lung disease and chronic steroid therapy and is now planned for TAVR with EVOLUT R CoreValve (34 mm) via percutaneous femoral access under conscious sedation.