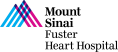Case and Plan:
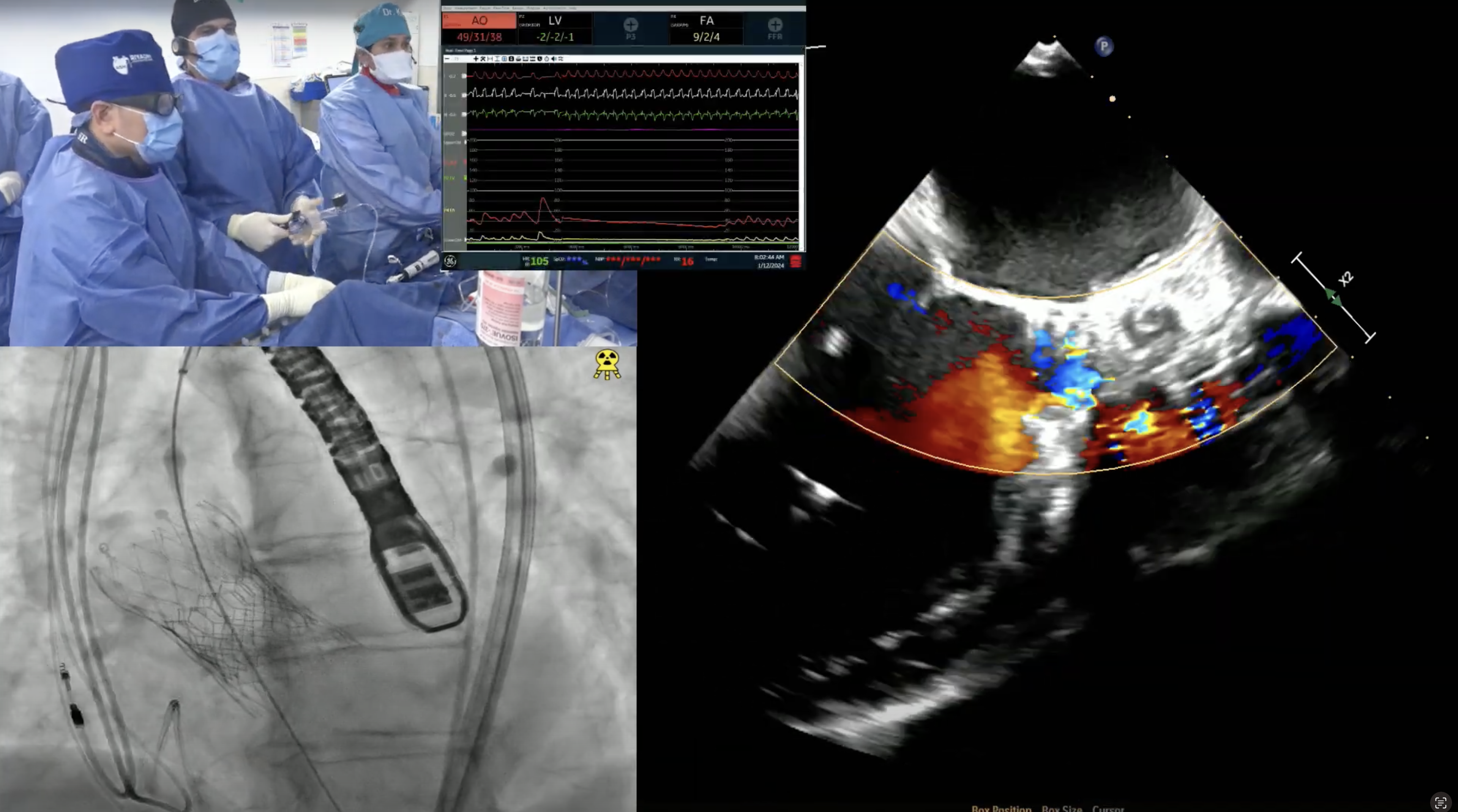
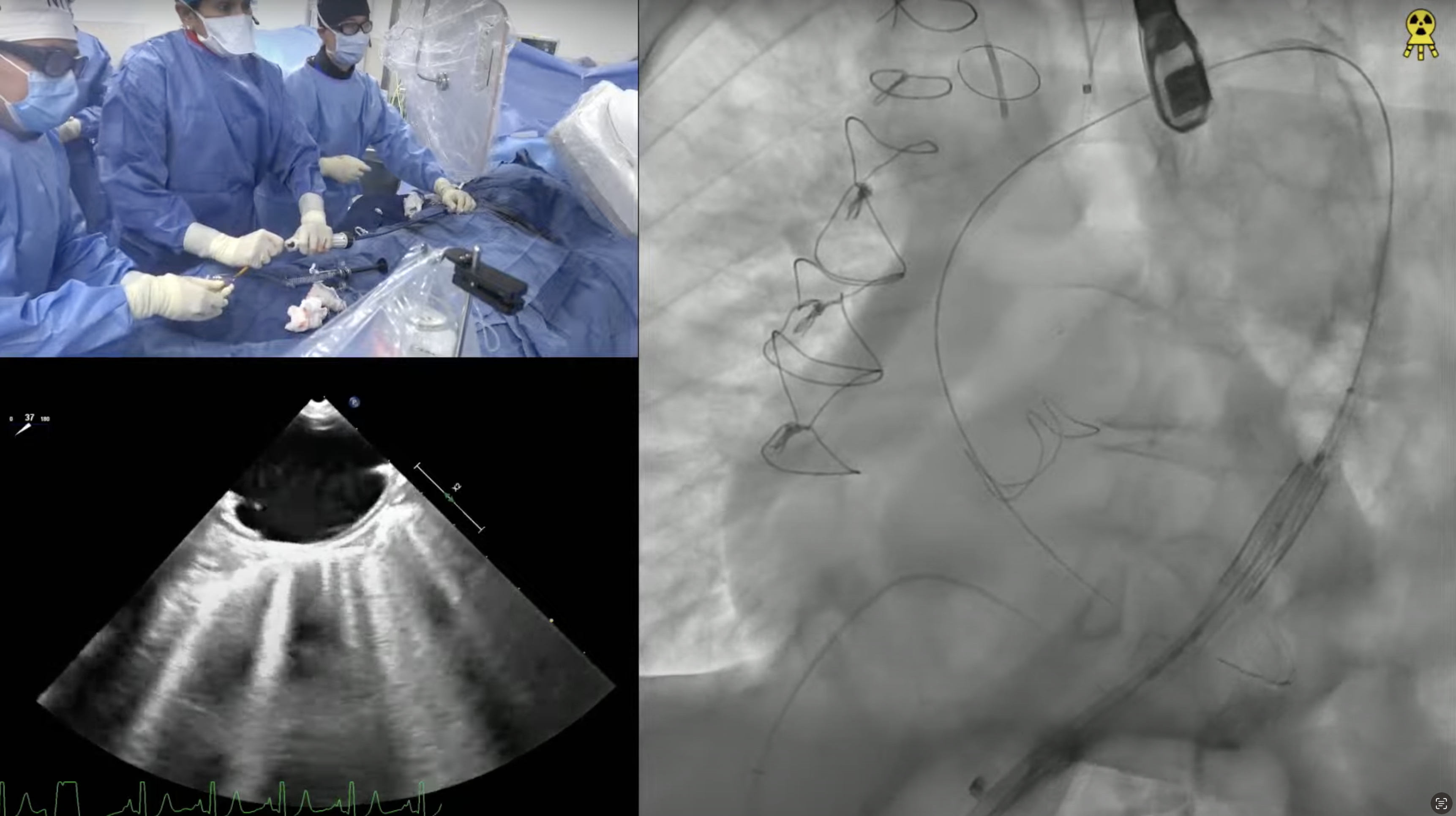
60 year-old male presents with worsening exertional dyspnea (NYHA Class III) and multiple admissions for CHF exacerbation in past 3 months. Past medical history is significant for symptomatic severe aortic insufficiency s/p bioprosthetic AVR (2017) with 23mm Magna 3000 valve, ESRD on HD, HTN and HLD. Recent echocardiogram revealed bioprosthetic valve degeneration with severe aortic stenosis (PG/MG/AVA of 75/51/0.67) and depressed LVEF of 30%. Coronary angiogram showed moderate 2V CAD being medically managed. Lower extremities on CT angiogram revealed calcified arteries with vessel size > 6mm. The Internal diameter of surgical AV measured 21.5×22.3mm (mean 21.9mm), perimeter 68.3mm and the area 370.6 mm2. The STS mortality risk for surgical AVR was calculated at 5.83%. The patient underwent Heart Team evaluation and was found to be at high risk for re-do SAVR due to comorbidities and frailty. Now planned for Valve-in-Valve TAVR with 26mm Evolut Pro+ CoreValve and bioprosthetic valve fracture using 23mm True Balloon via right percutaneous femoral approach.